- What is the Standard Two-Tiered Testing (STTT) algorithm for Lyme disease?
The STTT algorithm is depicted here.
- What is the ZEUS Modified Two-Tiered Testing (MTTT) algorithm for Lyme disease?
With ZEUS Borrelia MTTT™, by replacing the immunoblot with a second-tier ELISA assay, laboratories get rid of the technical complexities and inherent variability with blots.
ZEUS Scientific has established two algorithm options depicted in the flow charts below. The first algorithm, ZEUS Borrelia MTTT-1™, uses ZEUS ELISA™ Borrelia VlsE1/pepC10 IgG/IgM Test System as a first-tier test followed with ZEUS B. burgdorferi IgG/IgM Test System. The second algorithm, ZEUS Borrelia MTTT-2™, uses ZEUS ELISA™ Borrelia VlsE1/pepC10 IgG/IgM Test System as a first-tier test followed with ZEUS B. burgdorferi IgG Test System and ZEUS B. burgdorferi IgM Test System.
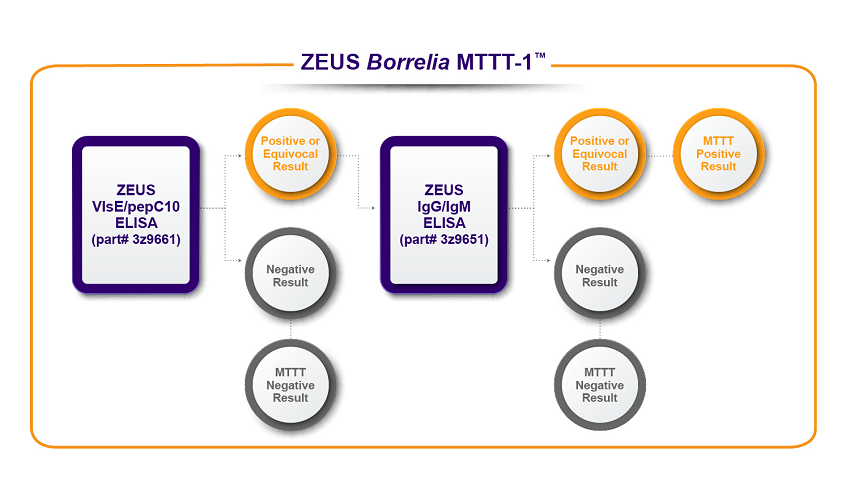
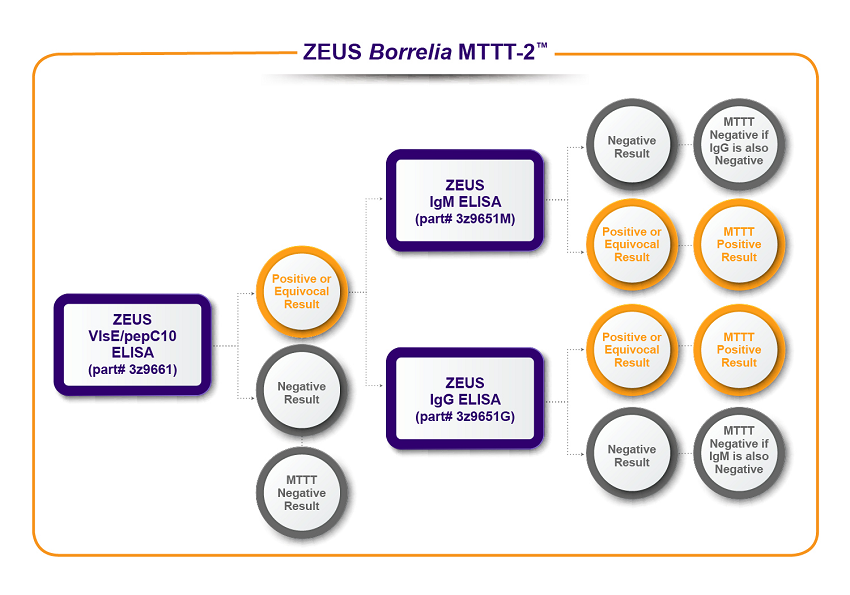
*Data on file. ZEUS Scientific
- Why did ZEUS Scientific pursue FDA-clearance of MTTT algorithms for the detection of Lyme disease?
A pioneer in Lyme disease testing, ZEUS Scientific was the first to receive U.S. FDA clearance for a serological test system to aid in the diagnosis of Lyme disease in 1989. Since then, ZEUS Scientific has continued to invest in and launch numerous best-in-class solutions for diagnostic laboratories around the world. Today, ZEUS is uniquely positioned to make the benefit-driven promise of a MTTT algorithm a reality in the clinical laboratory. We are ecstatic to have successfully addressed this longstanding diagnostic challenge by validating a Lyme disease testing approach that advances early detection capabilities by increasing sensitivity without negatively impacting specificity. This MTTT algorithm allows laboratories to simply and easily fully automate both steps of the Lyme testing algorithm, all with the benefit of detecting up to 30% more early Lyme cases*.
The significant results of our comprehensive clinical study exploring Modified Two-Tiered Testing (MTTT) algorithms versus the STTT algorithm are presented in the white paper; Validation of a Modified Two-Tiered Testing (MTTT) Algorithm for the Improved Diagnosis of Lyme Disease.
*Data on file, ZEUS Scientific.
- What are the results of the study comparing MTTT algorithms to the STTT algorithm?
Using a well-characterized retrospective cohort of Lyme disease and of healthy specimens as well as a large prospective cohort of routine patient samples, the clinical study demonstrated what the literature1,2,3 has repeatedly documented. A Modified Two-Tiered Testing (MTTT) algorithm of sequential ELISA tests is more sensitive in the serodiagnosis of Lyme disease than the Standard Two-Tiered Testing (STTT) algorithm with little change in the specificity.
Using ZEUS Scientific ELISA Borrelia tests, two separate protocols were found to be acceptable as MTTT algorithms:
- ZEUS Borrelia MTTT-1™: ZEUS ELISA™ Borrelia VlsE1/pepC10 IgG/IgM Test System followed by the ZEUS ELISA™ Borrelia burgdorferi IgG/IgM Test System.
- ZEUS Borrelia MTTT-2™: ZEUS ELISA™ Borrelia VlsE1/pepC10 IgG/IgM Test System followed by the combination of the ZEUS ELISA™ Borrelia burgdorferi IgG Test System and the ZEUS ELISA™ Borrelia burgdorferi IgM Test System.
The study found that either of these algorithm scenarios serves as a viable alternative to the STTT protocol that has been in place since 1994. Both MTTT scenarios are beneficial to clinical laboratories as they help to enable identification of cases of Lyme disease which may have been missed using the STTT protocol, especially Stage 1 and Stage 2 Lyme disease.
The results of our comprehensive study exploring MTTT algorithms versus the STTT algorithm have been presented in a white paper, Validation of a Modified Two-Tiered Testing (MTTT) Algorithm for the Improved Diagnosis of Lyme Disease.
Additionally, in a separate study utilizing a non-ZEUS screening assay within the STTT algorithm, ZEUS Borrelia MTTT™ detected up to 30% more Acute Lyme disease samples relative to the STTT*, significantly reducing the number of missed clinically positive patient samples. The results of this study are presented in our poster, Evaluation of several FDA-cleared Borrelia burgdorferi ELISAs within modified two-tiered testing algorithms.
*Data on file, ZEUS Scientific
- Branda JA, Linskey K, Kim Y, Steere AC, Ferrano MJ. Two-Tiered Antibody Testing for Lyme Disease With Use of 2 Enzyme Immunoassays, a Whole-Cell Sonicate Enzyme Immunoassay Followed by a VlsE C6 Peptide Enzyme Immunoassay. Clin Infect Dis 2011; 53:541–547.
- Mollins CR, Delorey MJ, Sexton C, Schriefer ME. Lyme Boreliosis Serology: Performance of Several Commonly Used Laboratory Diagnostic Tests and a Large Resource Panel of Well Characterized Patient Specimens. J Clin Microbiol 2016; 54:2726-2734.
- Branda JA, Body BA, Boyle J, Branson BM, Dattwyler RJ, Fikrig E, Gerald NJ, Gomes-Solecki M, Kintrup M, Ledizet M, Levin AE, Lewinski M, Liotta LA, Marques A, Mead PS, Mongodin EF, Pillai S, Rao P, Robinson WH, Roth KM, Schriefer ME, Slezak T, Snyder J, Steere AC, Witkowski J, Wong SJ, Schutzer SE. Advances in Serodiagnostic Testing for Lyme Disease Are at Hand. Clin Infect Dis 2018 Mar 19; 66 7):1133-1139
- Has the CDC shown its support for a Modified Two-Tiered Testing (MTTT) algorithm?
The CDC has acknowledged, through its own journal and in other publications including a major peer-reviewed publication for which experts from the CDC were co-authors,*,** that a Modified Two-Tiered Testing (MTTT) algorithm using an EIA instead of a second-tier Western immunoblot can be more sensitive in early Lyme than conventional (STTT) two-tier testing.1
The MTTT algorithm was noted as being easier to perform, eliminating the subjectivity of the second-tier Western immunoblots and providing improved sensivity.1 In the major peer-reviewed publication, it was stated that two-tier strategies involving two different EIAs perform as well or better than Standard Two-Tiered Testing (STTT) algorithms using EIA and Western blots.2
Assay developers were encouraged in the publication to submit data validating that their FDA-cleared assay, used as a second-tier test in place of immunoblotting, was substantially equivalent to, or better than, the current STTT algorithm.2
.
Finally, it is worth noting that following our FDA 510(k) clearance of the ZEUS Borrelia MTTT algorithms, the CDC promptly modified their website ( https://www.cdc.gov/lyme/diagnosistesting/index.html ) eliminating the traditional STTT flowchart and now recommends a “two-step process” making it clear that western or immunoblots are no longer required for the second step.
1Moore A, Nelson C, Molins C, Mead P, Schriefer M. Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of Lyme disease, United States. Emerg Infect Dis. 2016 Jul. http://dx.doi.org/10.3201/eid2207.151694 https://wwwnc.cdc.gov/eid/syn/en/article/22/7/15-1694.htm#r14
*Disclaimer: The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
2 Branda JA, Body BA, Boyle J, Branson BM, Dattwyler RJ, Fikrig E, Gerald NJ, Gomes-Solecki M, Kintrup M, Ledizet M, Levin AE, Lewinski M, Liotta LA, Marques A, Mead PS, Mongodin EF, Pillai S, Rao P, Robinson WH, Roth KM, Schriefer ME, Slezak T, Snyder J, Steere AC, Witkowski J, Wong SJ, Schutzer SE. Advances in Serodiagnostic Testing for Lyme Disease Are at Hand. Clin Infect Dis 2018 Mar 19;66 7:1133-1139. https://academic.oup.com/cid/article/66/7/1133/4706288
**The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. This publication is not an official US Food and Drug Administration guidance or policy statement. The meeting referred to in the text was supported by Cold Spring Harbor Laboratory Banbury Center, with a meeting grant from the Global Lyme Alliance. Meeting sponsors had no participation in the content of the meeting, or in preparation of the manuscript.
- How will using a MTTT algorithm make life in the laboratory better?
There are several operational advantages for the laboratory when using ZEUS Borrelia MTTT™, including:
- Fully automatable first-tier and second-tier workflow, not easily achievable with STTT
- Removal of subjectivity in reading Western and immunoblots
- Automation of time-intensive, error-prone manual tasks and elimination of any send-out expenses, improving turnaround time and cost efficiencies
Laboratories seeking ways to deliver both improved operational and clinical performance in detecting IgG/IgM antibodies to Borrelia burgdorferi can satisfy these objectives using ZEUS ZEUS Borrelia MTTT™ and ZEUS ELISA™ Borrelia Test Systems.
More information on our ELISA assays can be found here.
1Bacon R.,et al. 2003. Serodiagnosis of Lyme Disease by Kinetic Enzyme-Linked Immunosorbent Assay Using Recombinant VlsE1 or Peptide Antigens of Borrelia burgdorferi Compared with 2-Tiered Testing Using Whole-Cell Lysate. J Infect Dis. 187:1187-1199.
- Are ZEUS Scientific MTTT algorithms fully automatable?
Laboratories can choose the system that best fits their lab and workload: DS2®, DSX®, or Agility®. More information on the Dynex portfolio of instruments can be found here.
- What are thought leaders saying about MTTT?
Findings from these studies indicated that an EIA-based Modified Two-Tiered Testing (MTTT) algorithm for serologic diagnostic testing for Lyme disease may be a beneficial addition to the currently recommended STTT laboratory diagnostic algorithm.
Testing aspects valued by the publications’ authors include the simplicity of the MTTT algorithm and the removal of immunoblot subjectivity. Highlights from key publications include:
“This subjective reading and interpretation [of second-step immunoblots] can lead to erroneous positive results if weak bands are scored as positive in samples with negative enzyme immunoassay.”1
“Bacon et al. [ref] evaluated recombinant VlsE for detection of IgG and/or IgM antibodies, the peptide pepC10 for detection of IgM antibodies, and the peptide C6 for the detection of IgG antibodies, in comparison to the two-tier approach, for testing sera from 280 patients with LB. They analyzed the results of individual assays and of their potential combinations. The overall best sensitivity was seen with the combination of C6 IgG and pepC10 IgM compared with two-tier testing (78% versus 68%, respectively). The greatest difference was observed with sera of patients with EM; 63% of samples were positive for C6 IgG-pepC10 IgM during the acute phase, compared with 38% by two-tier testing.”2
“Although there were minor differences in sensitivity and specificity among MTTT protocols, each provides comparable or greater sensitivity in acute EM, and similar specificity compared with conventional 2-tiered testing, obviating the need for Western blots.”3
“In a retrospective investigation of patients referred to the private adult practice of an Infectious Diseases physician for possible for Lyme disease, 50 of 182 patients (27.5%, 95% CI: 21.1-34.6) were found to have a false positive IgM immunoblot. 78.0% of these patients had received unnecessary antibiotic therapy.”4
“Beyond improved sensitivity, the 2-EIA protocol offers several advantages compared with standard 2-tiered testing. The results are obtained objectively by an instrument system, and the information provided to the clinician is straightforward (i.e., the patient is either seropositive or seronegative), with an interpretation that is less complex than immunoblotting.”5
“Certainly, there is a downside to the 2-EIA algorithm, compared with Western blotting. With blots, one learns the spirochetal antigens against which the patient’s antibody response is directed... This gives information about the duration of illness... However, in routine cases, this level of detail is often superfluous and prone to misinterpretation.”6
- Aguero-Rosenfeld ME, Wormser GP 2015. Lyme disease: diagnostic issues and controversies. Expert Rev Mol Diagn 15:1–4. doi:10.1586/14737159.2015.989837.
- Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. 2005. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 18:484–509. doi:10.1128/CMR.18.3.484-509.2005.
- Branda, John A., Strle, Klemen, Nigrovic, Lise E., Lantos,Paul M., Lepore, Timothy J., Damle, Nitin S., Ferraro, Mary Jane, Steere, Allen C. Evaluation of Modified 2-Tiered Serodiagnostic Testing Algorithms for Early Lyme Disease. Clin Infect Dis. 2017 Apr 15; 64(8): 1074–1080.
- Seriburi V, Ndukwe N, Chang Z, Cox ME,Wormser GP. 2012. High frequency of false-positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 18:1236–1240. doi:10.1111/j.1469-0691.2011.03749.x.
- Branda JA, Body BA, Boyle J, Branson BM, Dattwyler RJ, Fikrig E, Gerald NJ, Gomes-Solecki M, Kintrup M, Ledizet M, Levin AE, Lewinski M, Liotta LA, Marques A, Mead PS, Mongodin EF, Pillai S, Rao P, Robinson WH, Roth KM, Schriefer ME, Slezak T, Snyder J, Steere AC, Witkowski J, Wong SJ, Schutzer SE. Advances in Serodiagnostic Testing for Lyme Disease Are at Hand. Clin Infect Dis 2018 Mar 19;66 7:1133-1139.
- Branda, J.A., Linskey, K., Kim, Y.A., Steere, A.C., and Ferraro, M.J. Two-Tiered Antibody Testing for Lyme Disease With Use of 2 Enzyme Immunoassays, a Whole-Cell Sonicate Enzyme Immunoassay Followed by a VlsE C6 Peptide Immunoassay Clin Infect Dis. 2011 Sep;53(6):541-7.
- What will be important to the lab’s physician customers when making a change from STTT to MTTT?
What will be most important to a laboratory’s physician customer is an understanding by the physician of the many benefits when moving from the STTT algorithm to the all-ELISA ZEUS Borrelia MTTT™, which include:
- Improved sensitivity in detecting early disease: In our recent clinical study using a well-characterized clinical cohort from the CDC, ZEUS Borrelia MTTT™ detected up to 30% more acute Lyme disease cases relative to the STTT algorithm*, significantly reducing the number of missed clinically diagnosed patient samples.
- Improved turnaround time: testing can now be performed in-house, eliminating the conducting of or sending out immunoblots, which may provide the physician with faster test results.
- Improved results reporting: physicians will be relieved of other immunoblotting results reporting drawbacks1 which include:
- Western blot testing may result in false-positive IgM testing2
- can be costly3
- understanding the antigens directed against the patient’s antibody response in routine cases is often unnecessary and prone to misinterpretation4
- STTT contains a gap in detecting 30+ days IgM+/IgG- Lyme positive samples under its IgM blot reporting requirements4
1Schoen, Robert T. Editorial Commentary: Better Laboratory Testing for Lyme Disease: No More Western Blot, Clinical Infectious Diseases, Volume 57, Issue 3, 1 August 2013, Pages 341–343.
2 Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme borreliosis, Clin Microbiol Rev , 2005, vol. 18 (pg. 484-509).
3 Microbiology Resource Committee, Tick-transmitted diseases survey TTD-B, 2010 Northfield, IL: College of American Pathologists.
4 Branda, J.A., Linskey, K., Kim, Y.A., Steere, A.C., and Ferraro, M.J. Two-Tiered Antibody Testing for Lyme Disease With Use of 2 Enzyme Immunoassays, a Whole-Cell Sonicate Enzyme Immunoassay Followed by a VlsE C6 Peptide Immunoassay Clin Infect Dis. 2011 Sep;53(6):541-7.
*Data on file, ZEUS Scientific
- I currently have an existing supply of ZEUS Borrelia Test Systems. Will I be able to use the kits that I have on hand within the MTTT algorithm, or will these kits now be obsolete?
The ZEUS ELISA™ Test Systems used in ZEUS Borrelia MTTT™ have not changed. If you have the following assays in stock, you can begin using them immediately in a MTTT fashion. The assays include:
- ZEUS ELISA™ Borrelia VlsE1/pepC10 IgG/IgM Test System (Part # 3Z9661)
- ZEUS ELISA™ Borrelia burgdorferi IgG/IgM Test System (Part # 3Z9651)
- ZEUS ELISA™ Borrelia burgdorferi IgM Test System (Part #3Z9651M)
- ZEUS ELISA™ Borrelia burgdorferi IgG Test System (Part # 3Z9651G)
The kits have not changed in any way with respect to their components or protocols. The only change is an intended use modification allowing for use in the MTTT algorithm. The assays are identical outside of this expanded intended use modification. Package inserts for each kit are available for download on the product web pages. Click on the products above to access the product web pages and links to the package inserts. To live chat or to speak with Technical Support, please visit https://www.zeusscientific.com/support or e-mail.
- How can I learn more about the MTTT algorithm?
We look forward to discussing how your lab can easily incorporate this novel algorithm and eliminate the time, cost and subjectivity associated with immunoblots while gaining valuable walkaway ELISA automation capability.
